(This blog is a short summary of the recently published review of Madani et al.,Cell Reprog. 2022 May 3, doi: 10.1089/cell.2022.0012. Online ahead of print.)
Introduction
As mentioned many times, the overall efficiency of IVF is stagnating or declining worldwide. Factors contributing to this disappointing tendency have been analysed in detail in the groundbreaking paper of Gleicher et al. (2019). The author listed several factors that may contribute to the disappointing outcomes. Among others, indiscriminate use of add-ons, including embryo selection methods, and controversial results with blastocyst cultures were also listed.
For the latter, great results achieved in the best clinics clearly demonstrate that the principle is correct, but lots of technical details are disregarded by many clinics, including the proper gas mixture, humidity, and consistent, undisturbed environment during the whole culture period.Unfortunately, the sharply decreasing basic research and minimal publication activity about technical issues also contribute to the lack of interest in improvement and lack of focus on details. Currently, almost all research related to embryo culture focuses on the selection of the best embryos for transfer while disregarding the fact that in a compromised embryo culture system, even the best embryos are handicapped.
In this unfortunate situation, decades may pass without a fresh, new idea, an alternative approach that may result in a much-needed breakthrough in the outcomes. Traditional methods, even if appropriately used (as said, far too frequently NOT used appropriately) have their limits. We should not accept these limits. We have to find a new, more efficient approach.
One possible alternative was outlined in the paper of Madani et al. (2022): in vitro culture of embryos from Day 1 to 5 without the zona pellucida.
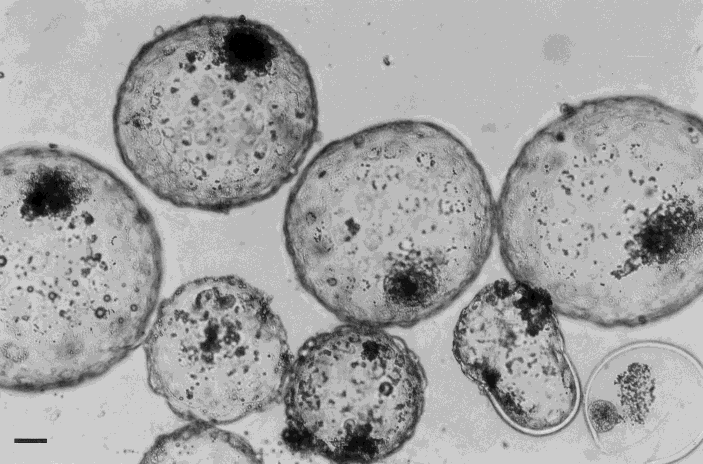
Bovine in vitro produced embryos on Day 8 after fertilisation
The role of the zona pellucida in vivo
The zona pellucida is an extracellular matrix that encapsulates the oocyte and the preimplantation stage embryo in humans and in most mammalian species. Although it contains no living cells and is seemingly passive, the structure of the zona pellucida differs in space and time. It consists of different layers, and its permeability and mechanical/biological resistance changes rapidly after fertilisation, then continuously during the preimplantation development.
Its main functions in embryo development are:
- Contribution to capacitation of sperm and protection of the oocyte from polyspermic fertilisation.
- Protection from mechanical damages; also from the aggregation of individual embryos to form chimaeras or disassemble blastomeres during the pre-compaction stage.
- Protection from harmful chemical and biological effects by selective permeability and forming a physical barrier against microbes.
- Protection of embryos from the attack of the maternal immune system during early development.
However, approaching the blastocyst stage, the role of the zona pellucida becomes redundant, eventually impeding implantation. "The Moor has done his duty; the Moor can go" (Schiller). Accordingly, due to the joint effort of enzymes of the uterine track and trophectodermal cells, the zona is peacefully dissolved and disappears without a trace.
Do we need the zona in vitro?
The tentative answer is: yes and no. Yes, before; and no, after fertilisation. However, the situation is a bit more nuanced.
Before fertilisation, we have very little information about the importance of the presence of zona. In domestic and experimental animals, zona-free oocytes (mostly as the result of a too aggressive collection and the handling procedure) are usually discarded. In humans, attempts may be made to save them. Successful fertilisations using both insemination and ICSI leading to healthy babies have been reported, but data are sporadic and do not allow scientifically sound conclusions. Obviously, zona-free oocytes are fragile, pipetting and ICSI mean a technical challenge, and embryologists do their best to keep the zona intact or just slightly damaged during ICSI.
After fertilisation, current embryo culture systems are based on zona-included embryos. Flat surfaces may result in the disaggregation of blastomeres; group cultures may allow aggregation and chimera formation. Since Louise Brown, we do our best to keep our embryos inside the zona till the expanded blastocyst stage, then watch happily their struggle to hatch with repeated expansions and collapse, as the previously used "assisted hatching" procedures don't seem to have any beneficial effects.
But is the zona really needed in vitro? For mechanical protection, semi-individual microwell cultures with appropriate size and shape (as the WOW dishes of VitaVitro) prevent mechanical damages, aggregation and disaggregation. Sterile culture media free of harmful agents (that may frequently be present in the maternal body and oviductal fluid) do not necessitate a biological or chemical barrier. The potentially harmful effect on zona early embryos - observed in experimental animals - is not a danger after blastocyst transfer.
But - why should we deal with zona-free embryo culture?
Is there any potential or proven benefit of this approach? Yes, it is.
Unfortunately, the zona pellucida does not seem to like the in vitro environment. The transitional hardening after sperm penetration in vivo becomes a constant and more drastic change in vitro. The whole structure of the zona is rearranged and becomes rigid and less permeable to chemical and biological agents. This may create problems during the whole culture period, with decreased nutrient supply and, even more importantly, decreased communication via growth factors and other ligands.
An additional but not smaller problem is the hatching. Instead of mild lysis, the zona stubbornly resists the attempts of the expanding embryos to escape, and the hard fight is energy-consuming in a difficult period when the embryo needs all its reserves to differentiate and implant. In a proper embryo culture system, most of them survive, but why make their - already challenging - in vitro life even more difficult?
Is there a way for harmless, rapid removal of the zona?
Yes, it is. A short exposition to pronase, then neutralising the enzyme with a protein-containing medium works with close to 100% efficiency. It has been used for millions of bovine and porcine embryos in our labs. Oocytes and embryos that resist this procedure (a handful out of a thousand) may be intrinsically developmentally handicapped. After a transitional deformation, all oocytes and embryos regain their original shape and proceed with normal development. The same approach was also successful in a few sporadic attempts with human samples.
What are the potential benefits?
In cattle, pig, sheep, buffalos, horses, etc., wherever the zona-free culture of handmade -cloned (HMC) embryos has been performed in WOWs, the development was impressing - comparable with the in vitro fertilised or parthenogenetically activated zona-intact counterparts. Zona-free cloned pig embryos had better development and higher cell number than traditionally cloned ones that were cultured inside the zona. After transfer, zona-free embryos had higher pregnancy rates, larger litters and healthier offspring than their traditionally cloned counterparts.
These are indirect pieces of evidence. No systematic comparison between human embryos cultured with or without the zona pellucida has been made yet.
However, we are talking about millions of domestic animal embryos produced without the zona; hundreds, rather thousands of offspring, and excellent outcomes. We agree that the radical change we suggest requires caution. Additionally, we may face legal and institutional barriers.
We do NOT suggest anybody break the law. However, reasonable new suggestions should find a way to get tested. Zona-free embryo culture from Day 1 to 5 in WOWs is a possible way to improve the quality of in vitro produced embryos, potentially leading to more successful cycles and more healthy babies. Stepwise, cautiously - yes. Still, we need to advance.

The WOW dish of VitaVitro
References
Madani, S., Machaty, Z., Vajta, G. An alternative way to improve mammalian embryo development in vitro: culture of zona pellucida-free embryos. Cell Reprog. 2022 May 3, doi:10.1089/cell.2022.0012. Online ahead of print.)
Gleicher, N., Kushnir, V.A., Barad, D.H. Worldwide decline of IVF birth rates and its probable causes. Hum. Reprod. Open, pp. 1-7, 2010 doi:10.1093/hropen/hoz017
For further references: see Madani et al., above.

